SAE Reconciliation
Open/Close sub menu
 eReconciliation®
Download the Solution Description
DOWNLOAD
GxP Compliance
Open/Close sub menu
eReconciliation®
Download the Solution Description
DOWNLOAD
GxP Compliance
Open/Close sub menu
KNOWLEDGE BASE
Reconciliation RegulationsReconciliation GlossaryReconciliation HandbookReconciliation ChecklistOUR EXPERTISE
Endpoint AdjudicationProtocol DeviationsData Safety Monitoring Boards eReconciliation Software Open/Close sub menuHOW WE HELP
Import & Compare SAE DatasetsAI Powered Records MatchingManage Reconciliation Operations With ActionsDetect and Manage SAE Data ChangesEnsure GxP Compliance with Audit TrailWHO WE HELP
CRO-Safe Partnership Program eReconciliation®
Download the Solution Description
DOWNLOAD
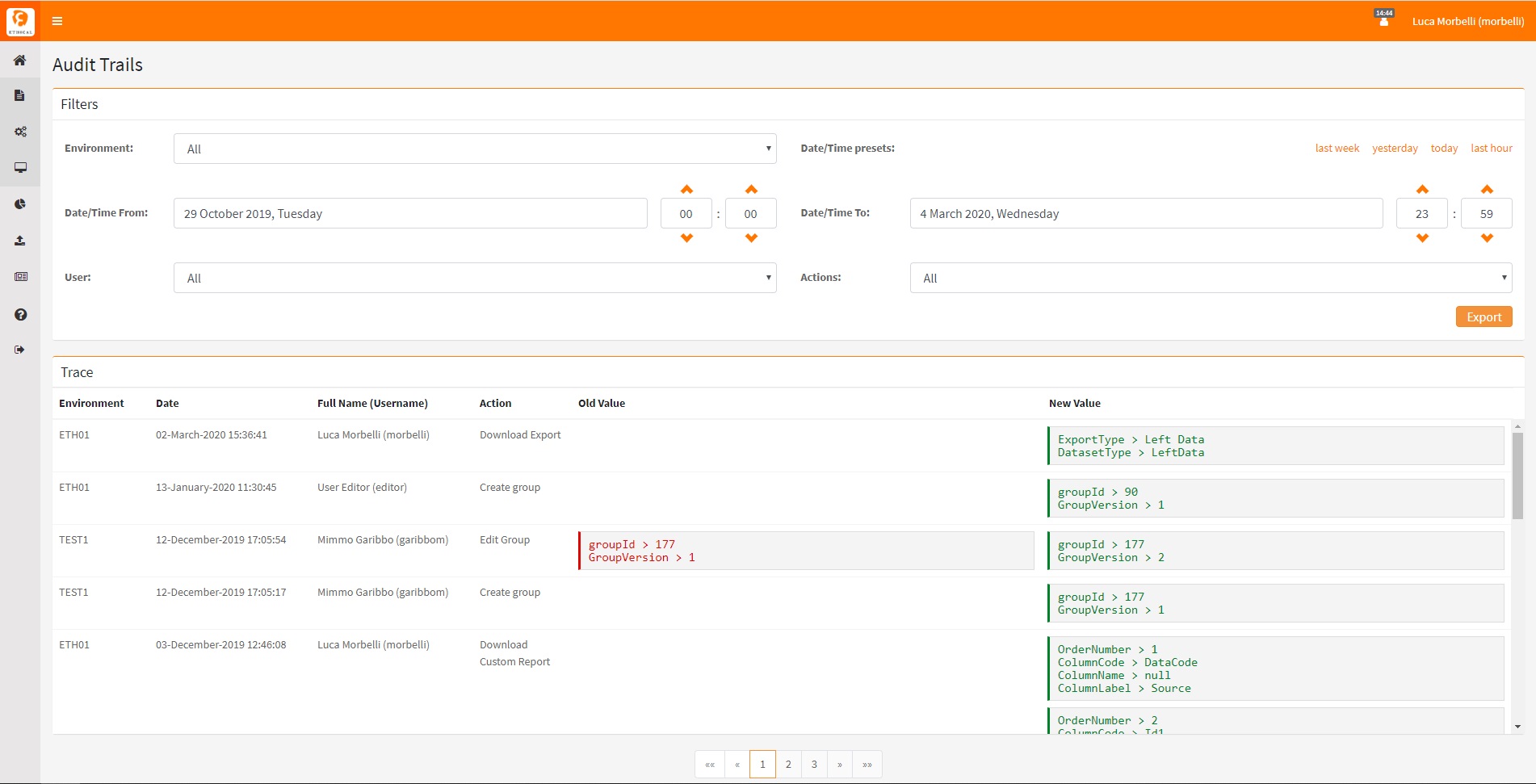
GxP Compliance
Open/Close sub menu
eReconciliation®
Download the Solution Description
DOWNLOAD
GxP Compliance
Open/Close sub menu